5-year results from a prospective, single-arm European trial on decellularized allografts for aortic valve replacement – The ARISE Study and ARISE Registry Data
Samir Sarikouch MD, PhD
Hannover Medical School, Department of Cardiothoracic Surgery, Hannover, Germany
Decellularized allografts (DAH) may provide an additional AVR option for very young patients as they can potentially overcome the high early failure rate of conventional allogenic and xenogeneic AVR prostheses. The near-normal haemodynamics in combination with the ability to repair a malfunctioning aortic root are especially important for patients with impaired myocardial function and patients with multiple previous aortic valve procedures.
In 2014, Hannover Medical School initiated a prospective multi-centre study (www.arise-clinicaltrial.eu), which was funded by the European Commission. The only prospective study performed to date on decellularized aortic homografts was registered under ClinicalTrials.gov, NCT02527629. Six tissue banks and 9 European hospitals participated, an innovative, Hannover-based, bio-tech company provided the decellularization service (www.corlife.eu).
144 patients (99 male) were prospectively enrolled in the ARISE Trial between 10/2015 and 10/2018 with a median age of 30.4 years (IQR 15.9-55.1). 28 % were paediatric patients and 45% of the patients had undergone previous cardiac operations. 19 % underwent two or more previous surgical procedures. In 24 patients (16.7 %) a prosthetic aortic valve was replaced with DAH. The mean implanted DAH diameter was 22.6±2.4 mm. The median operation duration was 312 min (IQR 234-417), the median cardio-pulmonary bypass time was 154 min (IQR 118-212), and the median cross-clamp time 121 min (IQR 93-150). No postoperative bypass grafting or renal replacement therapy was required.
After a median follow-up of 5.9 years (IQR 5.1-6.4, mean 5.5 ± 1.3 yrs. max. 7.6 yrs.), the primary haemodynamic endpoints peak gradient with median 11.0 mmHg (IQR 7.8-17.6) and regurgitation of median 0.5 (IQR 0-0.5) of Grade 0-3 were excellent.
At 5 years, freedom from death/reoperation/endocarditis/ bleeding/ thrombembolism were 97.9/93.5/96.4/99.2 and 99.3 % respectively. The 5-year results of the prospective multi-centre ARISE trial therefore continue to demonstrate DAH as safe for AVR with excellent haemodynamics. DAH results compared well with contemporary Ross cohorts despite more previous procedures in DAH patients, but showed more re-operations at 10 years within the ARISE registry.
Figure 1 shows data from the ARISE Registry for young adults (n=215) using decellularized aortic homografts (DAH) for aortic valve replacement in comparison to published meta-analysis data for several other options in young adults.
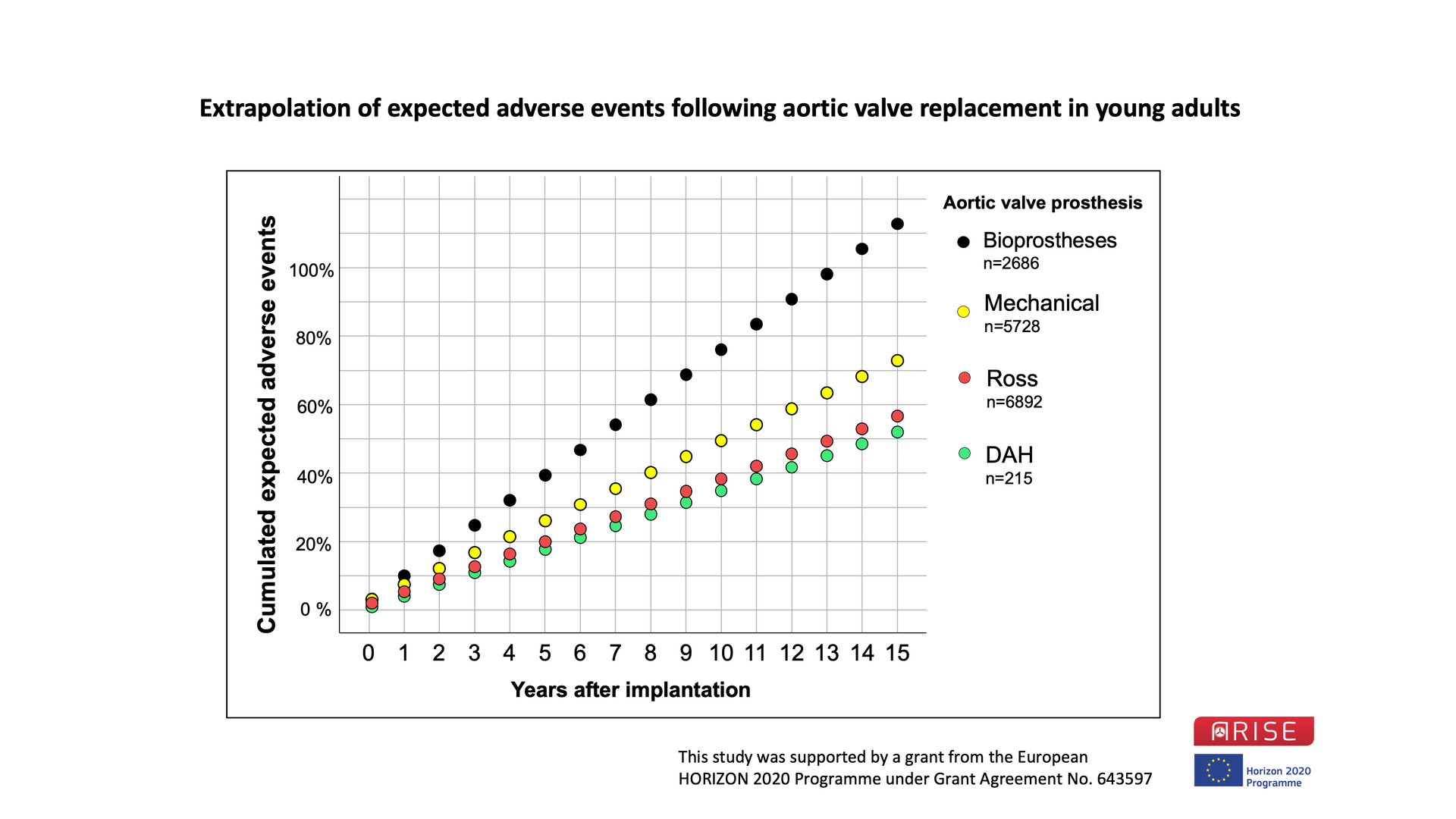
Figure 1: ARISE Registry Data of all 215 decellularized aortic homografts (DAH) implanted in young adults compared with recently published meta-analysis data from several AVR options in young adult patients. Perioperative and linearized annual adverse events such as death, reoperation or re-intervention, valve degeneration, thrombotic and bleeding events, and endocarditis were summarized to provide an estimate of adverse events in the long-term. While we followed multiple potential adverse events, the sum of these events could exceed 100 %.
Data taken from: Etnel et al. Circ Cardiovasc Qual Outcomes. 2019 Feb;12(2):e005481; Korteland et al. Eur Heart J. 2017 Dec 1;38(45):3370-3377; Etnel et al. Circ Cardiovasc Qual Outcomes. 2018 Dec;11(12):e004748.
Copyright © ARISE Hannover - Germany 2024 MH Hannover All rights reserved. | Impressum & Datenschutz
